PD Dr. med Bettina Baessler
Research group leader, Consultant radiologist
University of Zurich & University Hospital Zurich
About Myself
I am a consultant radiologist with special focus on quantitative and cardiovascular imaging including radiomics at the University of Zurich and the Department of Diagnostic and Interventional Radiology of the University Hospital Zurich.
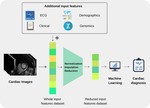
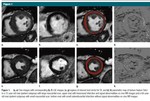
My special interest lies in the translation of quantitative imaging techniques to clinical routine and in the application of machine learning algorithms in medical imaging science. Over the past few years, my research has focused on the evaluation of the potential of radiomics and texture analysis combined with machine learning techniques for cardiac MRI. This work has led to an increasing awareness of the standardization issue in the field of radiomics. Currently, my group is working on various projects aiming to apply radiomics and deep learning to cardiac and oncological imaging, with a special focus on standardization. Since these emerging technologies require large datasets to accurately evaluate their performance, I was able to take lead in a large nationwide multicenter trial aiming at standardizing quantitative cardiac MRI.
It is my strong belief that research can only thrive through collaboration, hence my involvement in scientific societies such as the working group on cardiac imaging and of medical imaging IT of the German Radiological Society and the European Society of Medical Imaging Informatics (EuSoMII) has shown to be extremely rewarding.
No less important and rewarding for me is medical education, which has led me to develop and lead a quality improvement project for radiological teaching at my former department in Cologne, Germany. We completely overhauled teaching, providing lectures on demand and incorporating online voting systems during lectures. Since then, I have been involved in the development of the national training curriculum of medicine (Nationaler Kompetenzbasierter Lernzielkatalog Medizin, NKLM) as a speaker of the project group “digital compentencies” and as a co-speaker in the working group for the implementation of NKLM in Germany. The implementation of digital skills into the medical training curriculum is an extremely important topic, and I am excited about being part of this ongoing process.
Interests
- Cardiovascular imaging
- Quantitative MRI techniques
- T1 & T2 mapping
- Radiomics
- Advanced statistics
- Machine Learning
- Artificial Intelligence
Education
-
Habilitation (Priv.-Doz. / PD), 2018
University of Cologne
-
Medical Doctor (M.D.), 2011
University of Bonn
-
Medicine (Staatsexamen), 2010
University of Bonn